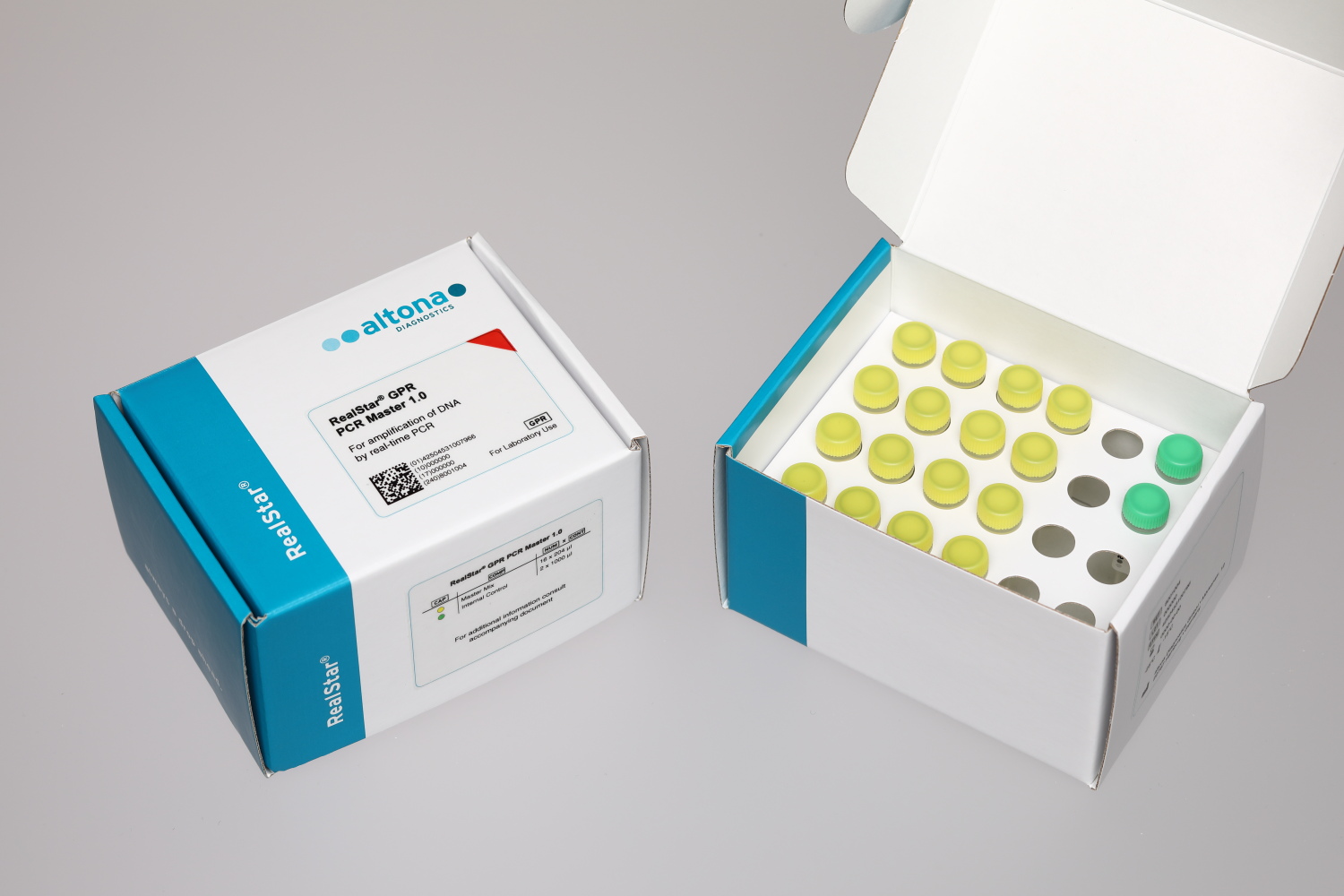
Intended use
The RealStar® GPR PCR Master 1.0 is a general purpose reagent (GPR) for the in vitro amplification and detection of target DNA by real-time polymerase chain reaction (PCR). The RealStar® GPR PCR Master 1.0 is not intended for a specific diagnostic application, but must be combined with appropriate analyte specific reagents (ASR) or other general purpose reagents to generate a finished in vitro diagnostic test.