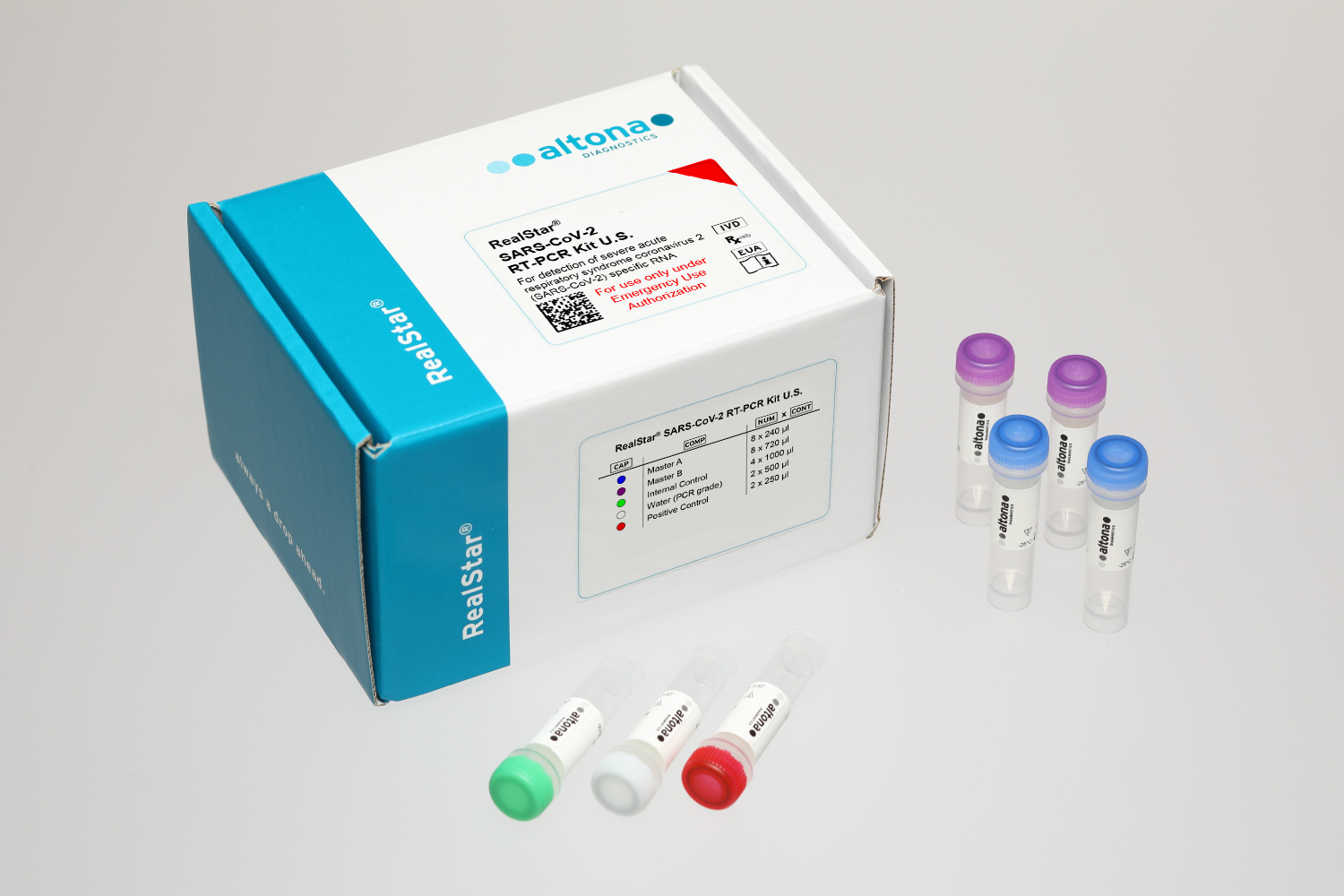
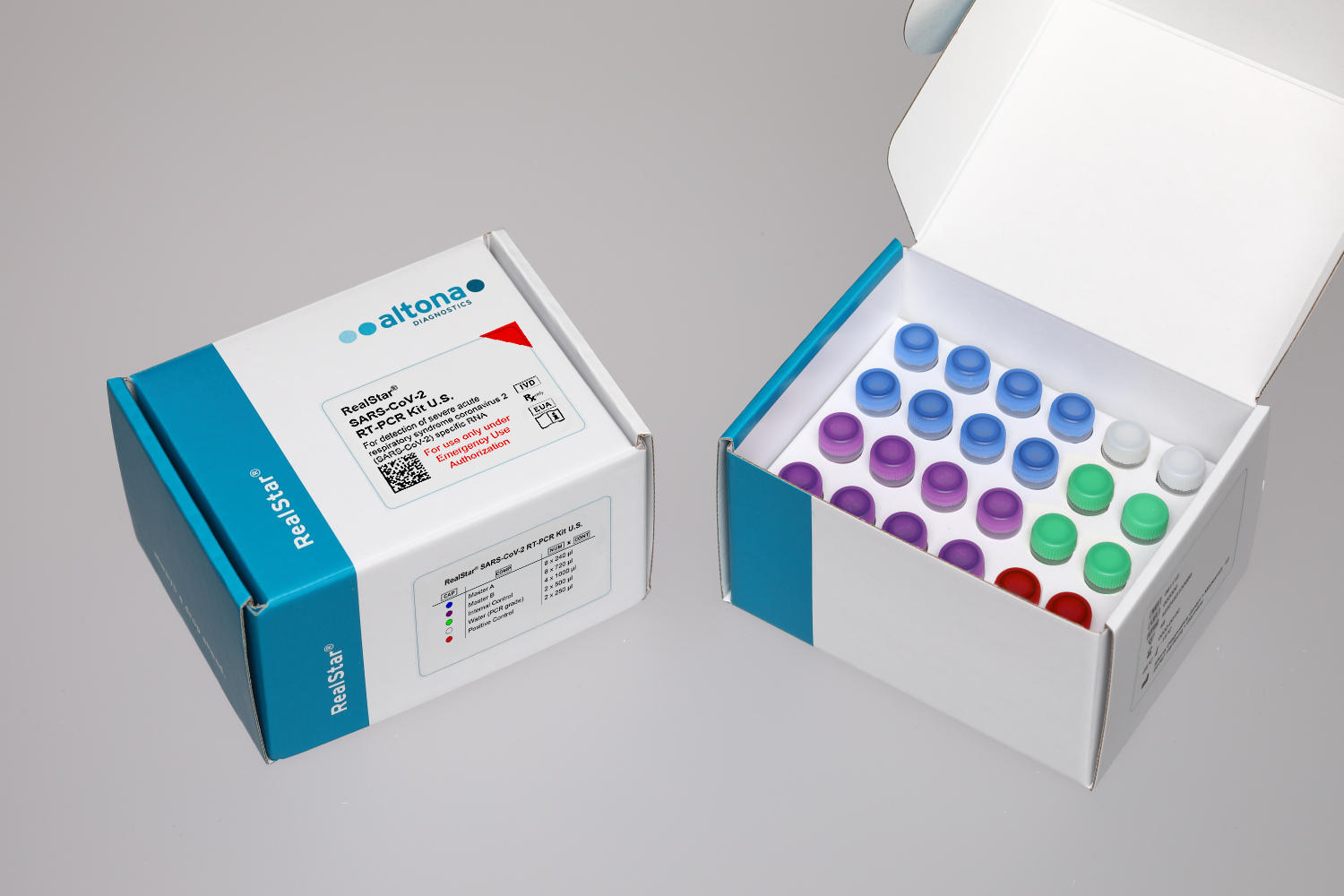
The RealStar® SARS-CoV-2 RT-PCR Kit U.S.* is authorized for a workflow consisting of nucleic acid extraction using the AltoStar® Automation System AM16 in combination with the AltoStar® Purification Kit 1.5 and the AltoStar® Internal Control 1.5, followed by the amplification and detection of SARS-CoV-2 specific RNA using the RealStar® SARS-CoV-2 RT-PCR Kit U.S.*.
Specifications
Sample types
Nasopharyngeal swabs
Oropharyngeal (throat) swabs
Anterior nasal swabs
Mid-turbinate nasal swabs
Nasal washes
Nasal aspirate
For use with
CFX96™ Touch Real-Time PCR Detection System
CFX96™ Touch Deep Well Real-Time PCR Detection System
Please remark
- This test has not been FDA-cleared or approved
- This test has been authorized by FDA under an EUA for use by authorized laboratories
- This test has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens
- This test is only authorized for the duration of declaration that circumstances exist justifying the authorization of the emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner