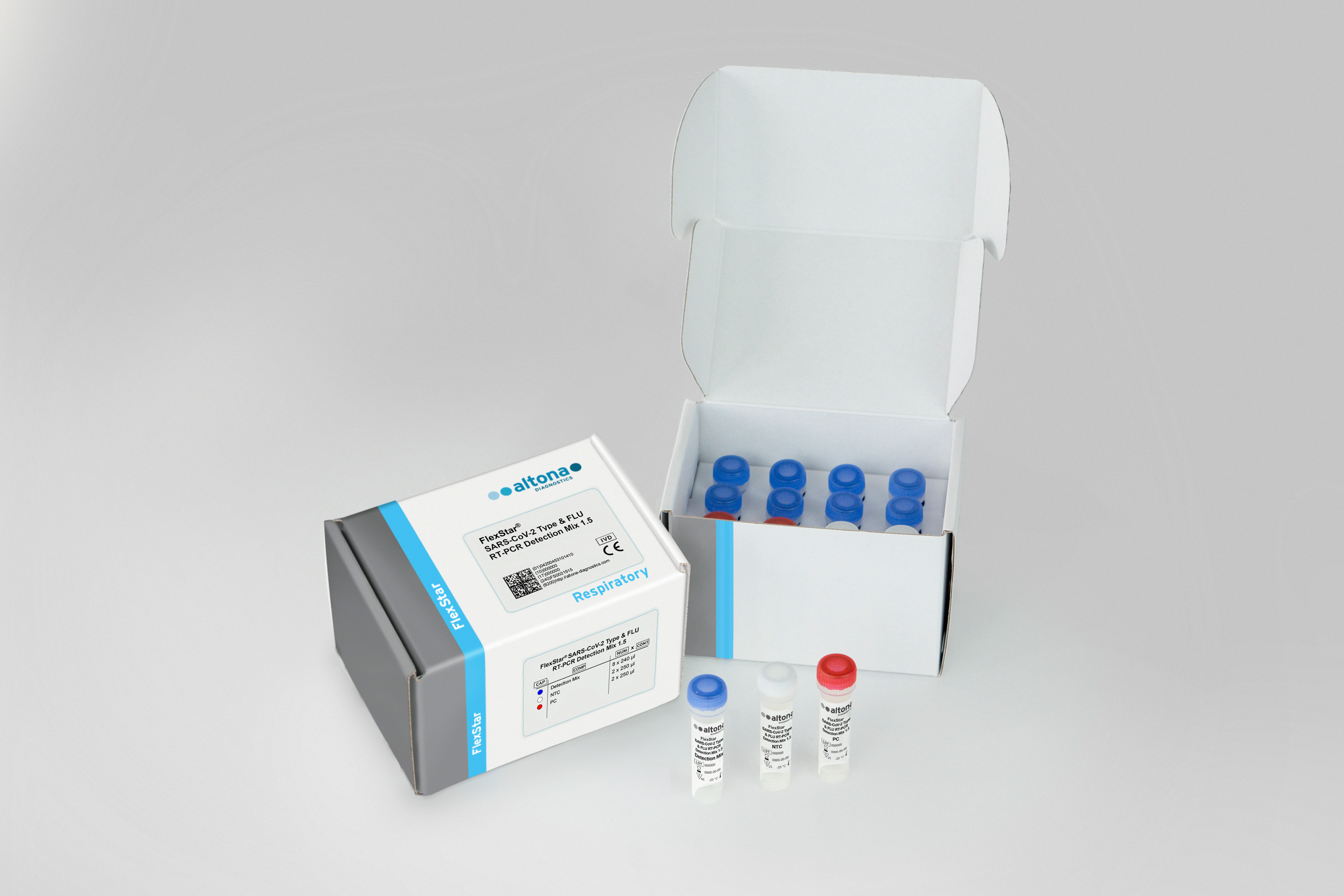
Intended use
The FlexStar® SARS-CoV-2 Type & FLU RT-PCR Detection Mix 1.5 is an in vitro diagnostic test, based on real-time PCR technology, for the qualitative detection and differentiation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza virus specific RNA in human respiratory swab specimens. The SARS-CoV-2 detection is based on the parallel detection of the E gene of lineage B-beta coronavirus (including SARS-CoV-2) and the S gene of SARS-CoV-2. Validated for use with the AltoStar® Automation System AM16 for nucleic acid extraction, the AltoStar® Internal Control 1.5 and the FlexStar® (RT-)PCR Amplification Mix 1.5.