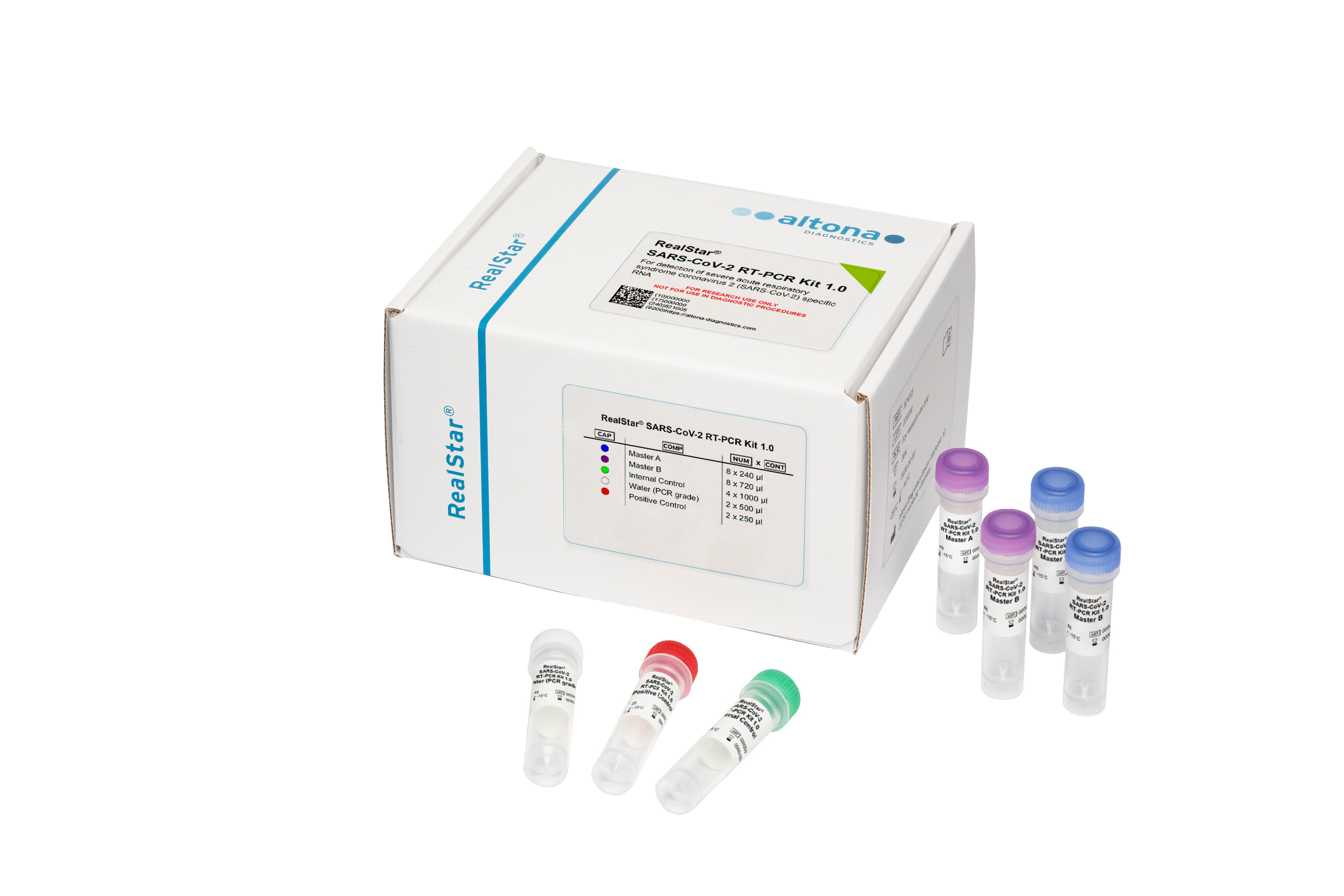
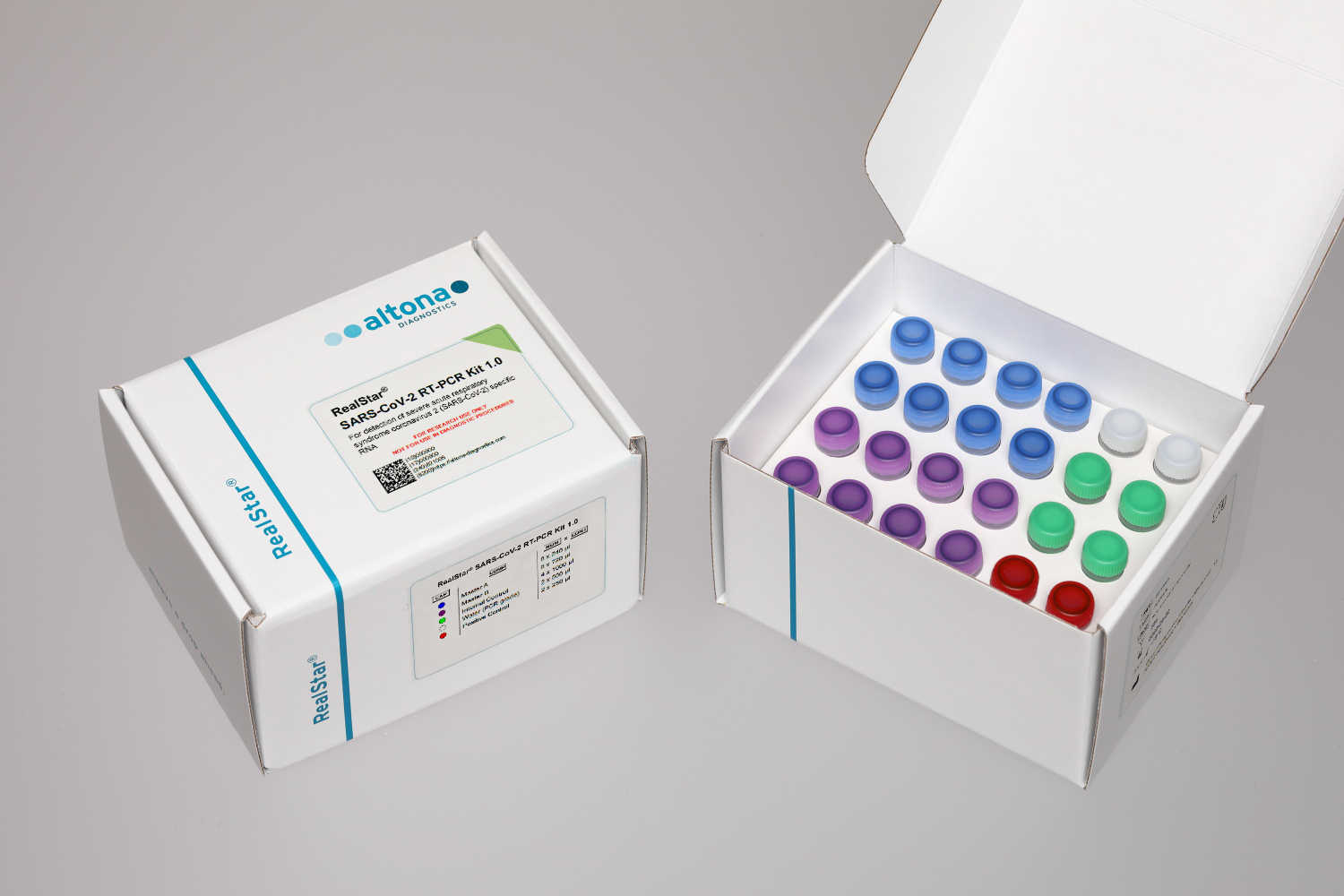
Application
The RealStar® SARS-CoV-2 RT-PCR Kit 1.0 is a reagent system, based on real-time PCR technology, for the qualitative detection of lineage B-betacoronavirus (B-βCoV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) specific RNA.
SARS-CoV-2, formerly known as 2019-nCoV, is the causative agent of the coronavirus disease 2019 (COVID-19).