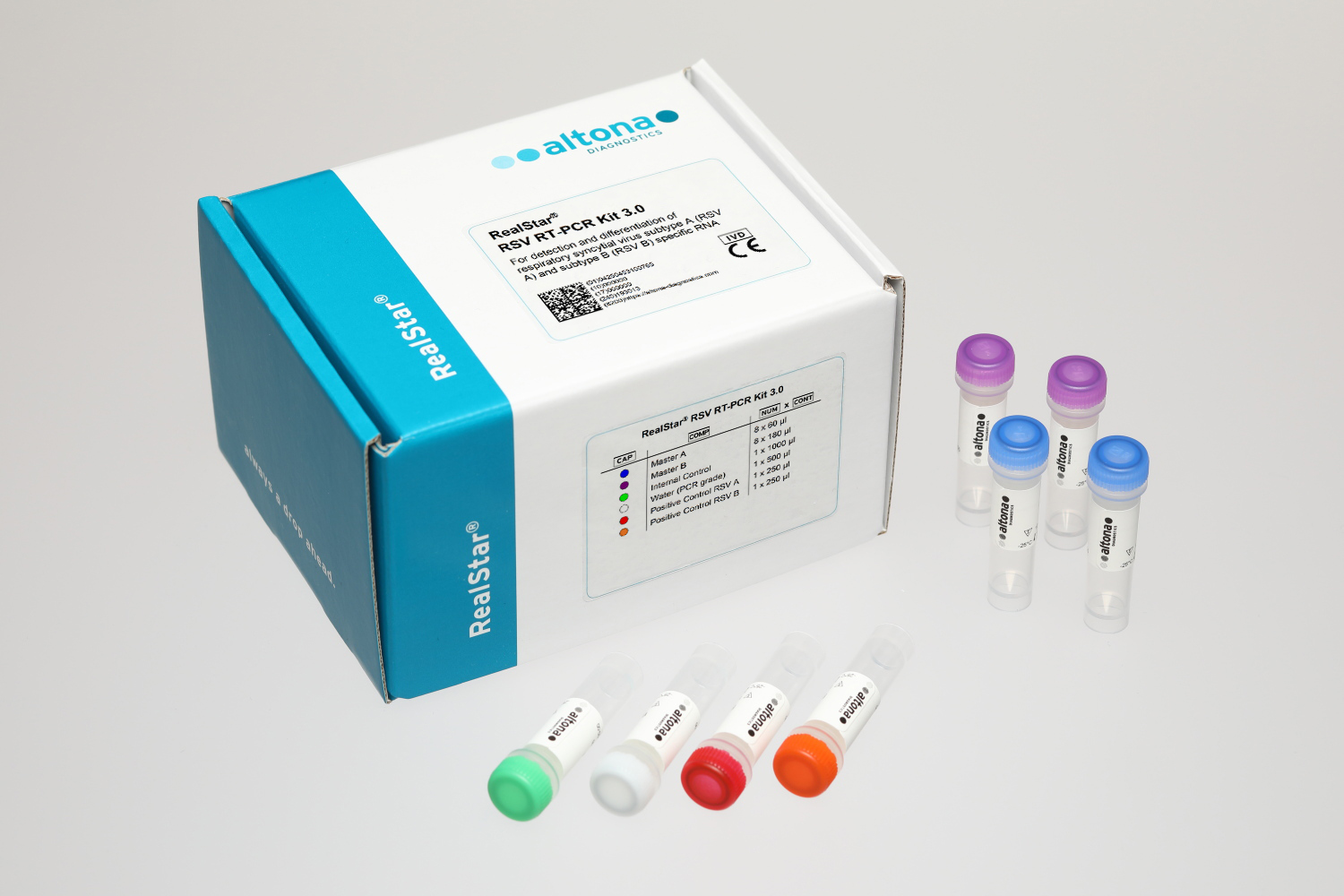
Intended use
The RealStar® RSV RT-PCR Kit 3.0 is an in vitro diagnostic test, based on real-time PCR technology, for the qualitative detection of respiratory syncytial virus (RSV) specific RNA. Furthermore, the test allows the differentiation between RSV subtype A (RSV A) and RSV subtype B (RSV B) specific RNA.